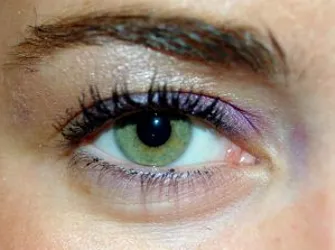
Growing Eyelashes The Lumigan Way
 The big news in the world of eyelashes is that the Food and Drug Administration (FDA) recently approved Bimatoprost (marketed as Lumigan) to grow eyelashes. Allergan, the maker of Botox will be releasing LATISSE™ which is their Lumigan based product in the Spring of 2009.
The big news in the world of eyelashes is that the Food and Drug Administration (FDA) recently approved Bimatoprost (marketed as Lumigan) to grow eyelashes. Allergan, the maker of Botox will be releasing LATISSE™ which is their Lumigan based product in the Spring of 2009.
(Image of Before And After Eyelashes From PRNews.wire.com - Before-After-Photo.pdf)
The cost is expected to be in the range of $120 for a month’s supply.
Available only through a doctor, LATISSE™ is a once-daily prescription treatment applied to the base of the upper eyelashes with a sterile, single-use-per-eye disposable applicator.
LATISSE™ users can expect to experience longer, fuller and darker eyelashes in as little as eight weeks, with full results in 16 weeks.
To maintain effect, continued treatment with LATISSE™ is required. If use of LATISSE™ is discontinued, eyelashes will gradually return to where they were prior to treatment over a period of weeks to months (average eyelash hair cycle).
Allergan has been using bimatoprost since 2001 in Lumigan, an Rx eye drop proven to lower eye pressure in people with glaucoma which can ultimately cause vision loss if too much pressure builds up. An interesting side effect of using Lumigan occurred in long time users. The users developed lush lashes, which prompted Allergan to look at marketing the Lumigan as a eye lash growth cosmetics product.
Lumigan is based upon bimotoprost which is a glaucoma drug that has been used by non glaucoma patients to grow eyelashes. Although Lumigan has been used by both RevitaLash and Jan Marini Age Intervention Eyelash, the folks at Allergen were the first to seek official FDA approval to use the drugs for cosmetic purposes.
Although its not exactly clear why and how Lumigan promotes eyelash growth, the Allergan folks have their own opinions of why.
Allergen has sued 11 companies for alleged patent infringement for using bimatropost to promote lash growth. Also, the FDA has previously seized eyelash products from Jan Marini Skin Research, Inc., of San Jose, California that were said to cause potential eye loss in users.
The Allergen lawsuit Jan Marini Skin Research, of San Jose, Calif., and three other companies were dropped after the companies in question acknowledged they had violated Allergan's patents on bimatropost and agreed to stop distributing products containing it.
FDA Special Warnings Concerning Lumigan:
Although the FDA has approved Lumigan to be used in future eyelash growth products produced by Allergan, the FDA has an entire list of warnings that come with Lumigan. These special warning include the following:
Lumigan may cause:
- Treated eye or eyes to change color (become more brown)
- Skin around the eye such as the eyelid to darken
- Eyelashes to increase in length, thickness, darkness and/or number
These changes happen slowly and may be permanent. The long-term effects of these changes are not known. Lumigan may also cause the middle part of the eye to swell and hold fluid (macular edema).
The most common side effects (not all are listed) of using Lumigan include:
- Red eyes
- Growth of eyelashes
- Itchy eyes
- Blurry vision
- Eye discomfort or pain
- Sensation of having an object in the eye
Social Media Network Information
Please follow us on Twitter at: https://Twitter.com/HairBoutique. I look forward to meeting new people from all walks of Twitter and learning from their Tweets.